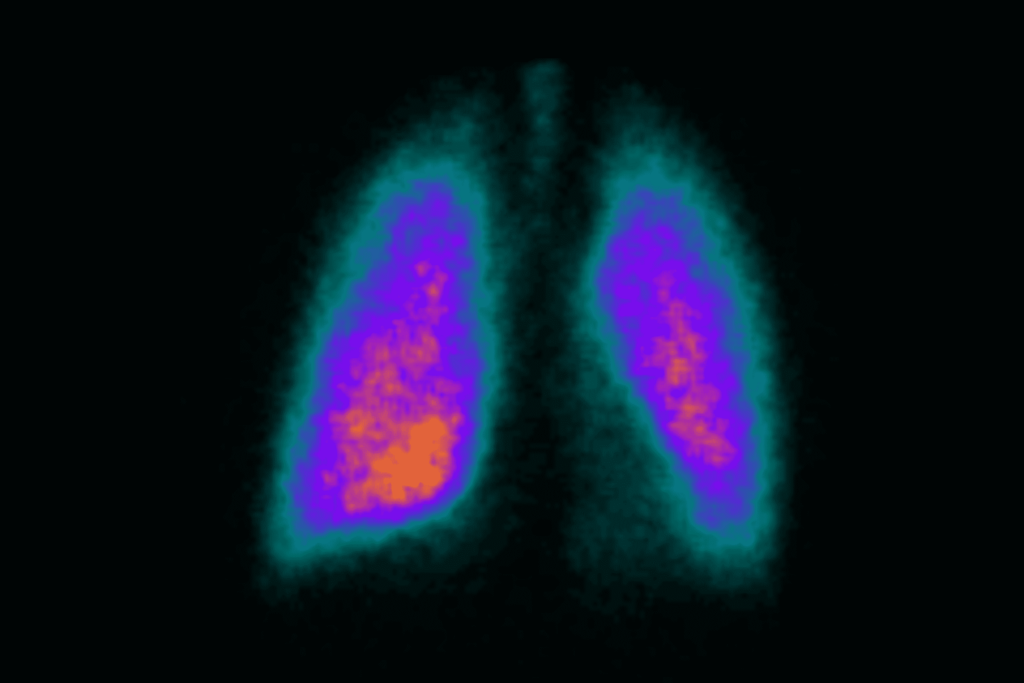
A scintigraphy study of budesonide/ glycopyrrolate/ formoterol fumarate metered dose inhaler in patients with moderate‑to‑very severe chronic obstructive pulmonary disease.
Simbec-Orion worked with two leading organisations, Astra Zeneca and Cardiff Scintigraphics, to successfully complete a scintigraphy study in COPD patients.
About the COPD study
Study Title
A scintigraphy study of budesonide/ glycopyrrolate/ formoterol fumarate metered dose inhaler in patients with moderate‑to‑very severe chronic obstructive pulmonary disease.
Aims and Process
Simbec-Orion was proud to partner with Astra Zeneca and Cardiff Scintigraphics to assess the pulmonary deposition of budesonide/ glycopyrrolate/ formoterol fumarate (BGF) in patients with chronic obstructive pulmonary disease (COPD).
This Phase 1 scintigraphy imaging study followed on from studies successfully completed with healthy volunteers. It aimed to assess the lung deposition of BGF administered via metered dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease.
Findings
The gamma scintigraphy methodology was applied to demonstrate that, even in patients with significantly compromised airways, BGF is deposited to the entire lung, including both small and large airways.
Read more about the gamma scintigraphy methodology and findings in the full report:
For more information on how Simbec-Orion can support your clinical trials on COPD or other studies, explore our website or contact us today.
Explore our related webinar – Scintigraphy: Providing Critical Data in Early Clinical Development