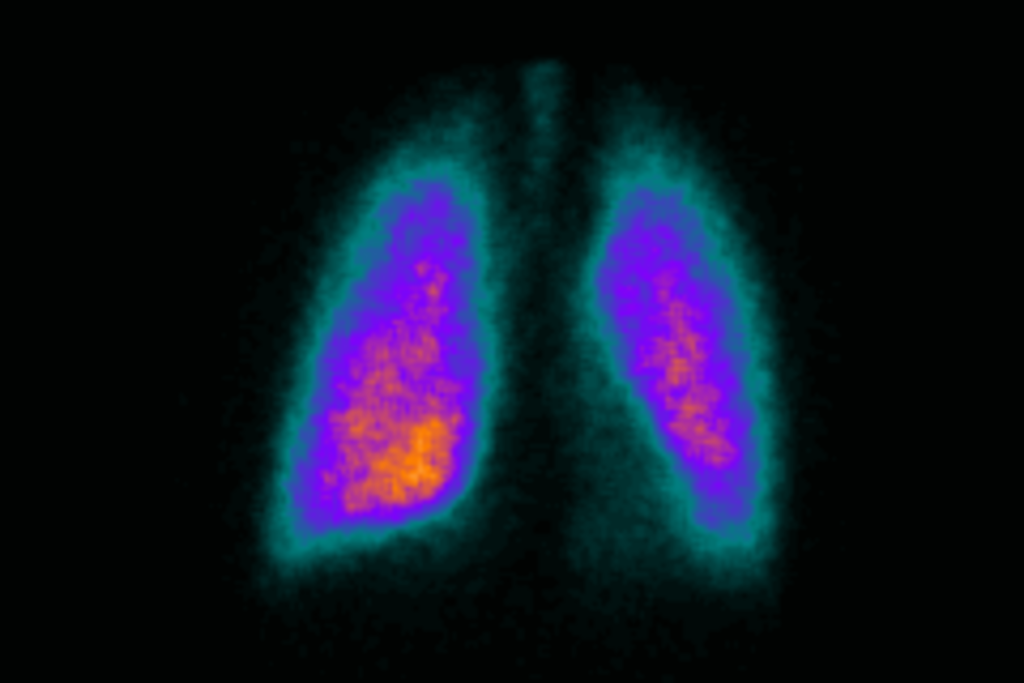
Gamma scintigraphy can play a vital role in drug product development, facilitating the determination of the biodistribution of dosage forms under physiological conditions. This information enables a detailed understanding of formulation and device variables and also the importance of patient factors. Thus scintigraphic data can inform critical decision making, thereby optimising development programs and improving cost-effectiveness.
We have been working in partnership with gamma scintigraphy specialists, Cardiff Scintigraphics Limited, for over 25 years enabling us to provide seamless scintigraphy services for our clients.

Leveraging scintigraphy in a Phase I patient study
We partnered with a large pharmaceutical Sponsor and our colleagues at Cardiff Scintigraphics Ltd to demonstrate that a novel, triple combination pMDI formulation could effectively deliver drugs to the lungs of patients with moderate and severe/very severe COPD.
Determining biodistribution of pharmaceutical dosage forms
During the clinical study, gamma scintigraphy can be employed using our on-site dedicated dual head gamma camera to evaluate deposition, residence, clearance, and dispersion of pharmaceutical dosage forms in healthy volunteers and patients.
Our approach is tailored to the specific objectives of the study, conducting planar scintigraphy scans to non-invasively assess regional or whole body radiolabel distribution. Analysis of the images enables the deposition, residence, clearance and dispersion of the dosage form to be characterised.
The scintigraphic analysis can also be complemented with expertise in pharmacokinetic and pharmacodynamic evaluations, drug assay development and bioanalysis.
The scintigraphy process
The scintigraphy process involves direct or indirect radiolabelling of a selected component of the study dosage form using a short-lived gamma emitting radioisotope e.g. technetium-99m or indium-111. The radiolabelling procedure undergoes rigorous validation prior to starting the study.
The radiolabelled trial dosage form has a short shelf-life and is released by the QPs in our GMP facility on the day of dosing. A critical success factor is ensuring that the protocols are designed, and logistics of each dosing day carefully planned, to deliver high-quality results. Our team understands the practicalities and intricacies of conducting gamma scintigraphy studies and will advise on the best approach.
Characterising drug delivery
Gamma scintigraphy is routinely used to characterise drug delivery from oral dosage forms, factors such as gastric residence/emptying, gastrointestinal transit and the site of disintegration/dispersion can be determined.
In the respiratory tract, the performance of inhaler systems is evaluated by determining total lung deposition and the fraction deposited in the oropharynx and subsequently swallowed, the exhaled fraction is also measured. Regional distribution within the central and peripheral regions of the lung can also be quantified. Subjects routinely undergo gas ventilation images (Krypton-81m) to ensure accurate derivation of the lung margins and central and peripheral regions. Furthermore, physiological functions such as mucociliary clearance may be directly studied using this technique.
Simbec-Orion recently worked with Astra Zeneca and Cardiff Scintigraphics, to successfully complete a scintigraphy study in COPD patients.
Read more here.
Full image analysis
The team will provide an end-to-end expert scintigraphy service, with full image analysis and data interpretation for clinical trial reporting. All our data processing methods are verified and accurate corrections for tissue attenuation in different anatomical regions are calculated, from transmission images, on a subject-by-subject basis.
A complete electronic data trail from raw image file to attenuation, decay and background corrected data forms part of our standard reporting procedure. Our thorough process works to deliver the best possible clinical outcome.
What our
Clients Say About Us
The people working at Simbec Orion are dedicated, very professional and they cover all aspects of clinical research. A team will be set up for your project, who will have a strong focus on your study in a very open minded and friendly atmosphere. The studies I have outsourced to Simbec Orion, have been successfully managed and performed. I will use Simbec Orion in the future and I can strongly recommend them to other pharmaceutical companies.
Tina Lidén Mascher, Head of Clinical Operations
Klaria AB