Specialist CNS endpoints
Historically, clinical trials for central nervous system (CNS) conditions were challenging for a multitude of reasons, with a failure rate for new drugs being very high relative to other therapeutic areas. Using a variety of neuroscience tools and techniques at the Phase I or first-in-human (FIH) phase of the drug development pipeline, it is now possible to gather the data and insights needed to build a detailed picture of the CNS effects of drug candidates which can help to build a business case for future CNS clinical trials.
This means that the development of your therapeutic for a CNS indication requires access to specialist support from your Contract Research Organisation. Simbec-Orion has decades of experience supporting the development of assets in this important therapeutic area. From Phase I healthy volunteers through to later phase multi-centre trials, our capability includes therapeutics for neurodegenerative, psychiatric and autism spectrum disorders, as well as analgesics and more.
Early phase studies
First in human studies are conducted in our MHRA-accredited Clinical Pharmacology Unit in Wales, UK. This purpose-built facility includes on-site IMP management and central laboratories to ensure the smooth and efficient conduct of your study. An established relationship with the local Research and Ethics Committee assists in faster turnaround and approval decisions.
Case Study
Cardiff University MDI-26478 drug is a potential new treatment for schizophrenia developed by the Medicines Discovery Institute (MDI) at the university.
Our scientists worked with their colleagues at the MDI to design a three-part First in Human study. This required us to collaborate with both the Cardiff University Brain Research Imaging Centre (CUBRIC) for neuroimaging and cognitive assessments, as well as niche CRO, The Science Behind, who performed the EEG assessments, and collected CSF samples in our Clinical Pharmacology Unit.
The Science Behind
Based in Cardiff, less than an hour from our Clinical Pharmacology Unit, our clients have access to additional specialist services through our established partnership with our colleagues at The Science Behind.
This niche CRO combines neuroscience, neurology and psychology expertise with access to hardware, to bring innovative methods for studying the effects of CNS-active drugs in early-phase clinical trials.
Cardiff University Brain Research Imaging Centre
Located just a short drive from our facility, we also partner with our colleagues at the Cardiff University Brain Research Imaging Centre (CUBRIC).
Bringing together world-leading expertise in brain mapping and the very latest in brain imaging and stimulation, this facility provides us with access to a powerful combination of imaging technology and scientific excellence.
Featuring four Magnetic Resonance Imaging (MRI) systems as well as a Magnetoencephalography (MEG) laboratory, our collaboration allows us to apply cutting-edge methodology to clinical trials for new CNS therapeutics.
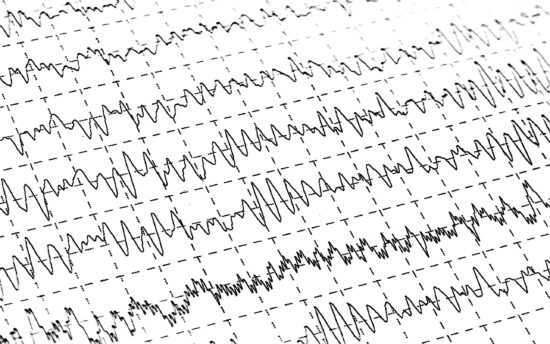
Speciality services for CNS indications include:
- CSF sampling. Cerebral Spinal Fluid (CSF) can be an indicator of blood-brain barrier penetration as well as being a useful source of several biomarkers for disease and/or drug. Changes in these biomarkers therefore could demonstrate pharmacodynamic effect. Specialist medical staff are on-site, trained in the collection of cerebrospinal fluid.
- Transcranial Magnetic Stimulation (TMS) Provided in conjunction with our colleagues at The Science Behind, TMS combined with EEG/EMG is an innovative technique that uses pulsing magnetic fields to activate or suppress brain activity.
- Electroencephalography (EEG) Also available through our collaboration with The Science Behind, this technique is useful for safety monitoring, but can also be used independently or with other techniques (like TMS or cognitive measurements) to measure drug effect and dose-response.

Multi-centre phase I trials
In addition to our purpose build unit, we can also coordinate multi-centre phase I trials, where appropriate for the protocol and patient group. This form of clinical trial can remove roadblocks in participation such as travel times, cost, and inconvenience, making patient recruitment smoother.
We have established links with other Phase I CROs to facilitate larger, multi-centre studies.
Indication experience
- Autism spectrum
- Alzheimer’s Disease
- Huntington’s Disease
- Parkinson’s Disease
- Anxiety
- Depression
- Schizophrenia
- Migraine
- Pain, including chronic pain
Later phase development
Our CNS experience extends to support of later phase clinical development with study designs including food effect, bioequivalence, as well as absolute and comparative bioavailability.