Understanding respiratory disease trials
Chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and asthma remain a leading cause of death and disability across the globe. According to WHO, COPD was 4th cause of death, causing 3.5 million deaths in 2021, approximately 5% of all global deaths1. In 2017, nearly 545 million people worldwide were living with some form of chronic respiratory disease, which represented a 39.8% increase when compared with the incidence in 19902.
The incidences of other respiratory diseases such as Idiopathic Pulmonary Fibrosis (IPF) are also on the increase, with prevalence ranging from 0.33 to 4.51 per 10,000 depending on geographical population3.
Clinical trials for novel therapeutics to treat this growing patient population require specialist experience and knowledge.
Early phase studies
Leveraging our location within a geography where 1 in 5 people are diagnosed with a lung condition at some point in their lives2, Simbec-Orion conducts early phase studies in our MHRA-accredited Clinical Pharmacology Unit. With nearly five decades of experience in conducting studies including multiple inhaled delivery types such as pressurised metered dose inhalers (pMDIs,) dry powder inhalers (DPIs), and nebulisers, this purpose-built facility comprises on-site spirometry capability, IMP management, and central laboratories to ensure smooth and efficient conduct throughout. An established relationship with the local Research and Ethics Committee assists in faster turnaround and approval decisions.
Indication experience
- Asthma
- COPD
- Pneumonia (inc. ventilator associated pneumonia)
- Emphysema
- COVID 19
- Lung Injury
- Cystic Fibrosis
- Idiopathic Pulmonary Fibrosis (IPF)
Specialist services for respiratory indications
- Respiratory chambers
- Scintigraphy
- IMP manufacture experience with solutions for inhalation/nebulisation including radiolabelled pMDIs, DPIs and nebuliser systems
- Sputum induction

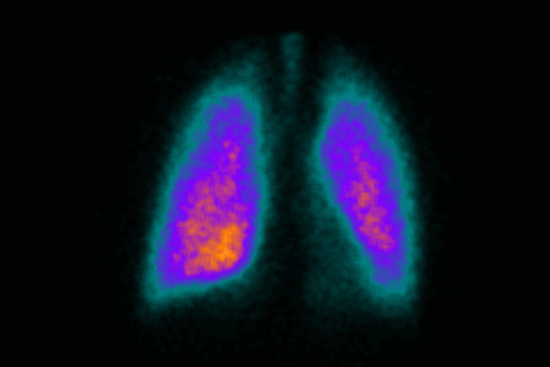
Collaboration with Cardiff Scintigraphics
Gamma scintigraphy can play a vital part in the drug development process, used to determine the biodistribution of drug delivery systems under physiological conditions and can be especially useful to determine the deposition of inhaled IMP.
We have been working in partnership with gamma scintigraphy specialists, Cardiff Scintigraphics Limited, for over 25 years enabling us to provide seamless scintigraphy services for our clients as part of their clinical trial.
Mucociliary clearance
The European Commission aims to phase out the hydrofluorocarbons (HFCs) currently used as excipients/propellants in pMDIs i.e. HFA134a and HFA227ea, in favour of propellants of low global warming potential. Indeed, many companies have already taken the initiative and requested scientific advice on switching to such propellants in their existing formulations. A major change to the formulation, such as this, requires data not only confirming that the proposed replacement is safe and non-toxic, but also that there is no impact on the efficacy and deliverability of the active substance in the final formulation. Simbec-Orion, in conjunction with our colleagues at Cardiff Scintigraphics Limited, can design and deliver a study design that will fully support your regulatory need for robust bioequivalence data.
Multi-centre phase I trials
In addition to our purpose build unit, we can also coordinate multi-centre phase I trials, where appropriate for the protocol and patient group. This form of clinical trial can remove roadblocks in participation such as travel times, cost, and inconvenience, making patient recruitment smoother.
We also have established links with other Phase I CROs to facilitate larger, multi-centre studies.

Later phase development
Our respiratory experience extends into later phase clinical development with multi-centre clinical trials across different geographical regions. These studies can be further enhanced with the inclusion of food effect, bioequivalence, as well as absolute and comparative bioavailability studies conducted as part of your late-phase development program.
Adaptive Trial Design for Novel IPF Small Molecule Therapy: A case study
A small biotech client had developed an asset that was a potential treatment for Idiopathic Pulmonary Fibrosis (IPF). Simbec-Orion was able to provide regulatory advice and support for submission to the MHRA, EMA and FDA, along with a study program that accelerated the delivery of their studies, saved cost and facilitated the transition from Healthy Volunteers (HV) to Phase II trials.
References
1. WHO website https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) accessed February 27, 2025
2. GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020 Jun;8(6):585-596. doi: 10.1016/S2213-2600(20)30105-3. PMID: 32526187; PMCID: PMC7284317.
3. Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, Patel H, Kreuter M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021 Jul 7;22(1):197. doi: 10.1186/s12931-021-01791-z. PMID: 34233665; PMCID: PMC8261998.
4. Asthma and Lung UK website: https://www.asthmaandlung.org.uk/wales, accessed February 27, 2025




