A global CRO
with a responsive, flexible approach
Early to late phase clinical trials. Delivered.
We are Simbec-Orion, who for almost five decades, have been delivering clinical trial management services across a wide range of therapeutic indications and phases. A clinical research organisation with a flexible, specialist approach, we strive to become a trusted partner for our clients. Our passion as a CRO is rare diseases and oncology.


Early to late phase clinical trials. Delivered.
We are Simbec-Orion, who for almost five decades, have been delivering clinical trial management services across a wide range of therapeutic indications and phases. A clinical research organisation with a flexible, specialist approach, we strive to become a trusted partner for our clients. Our passion as a CRO is rare diseases and oncology.

Early regulatory support
The value of Early Regulatory Engagement: How Scientific Advice Can Support your Clinical Development
In this webinar, Dr Kirsty Wydenbach, a valued member of our independent Drug Development Advisory Board and Head of Regulatory Strategy at Weatherden, shares her insights on the benefits of early, proactive engagement with regulators through Regulatory Scientific Advice.


Defined by experience
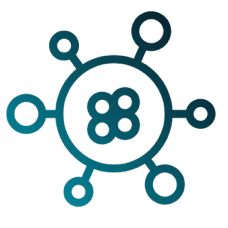
Responding to the evolving needs of our clients has made us the global contract research organisation we are today. Offering a full-service clinical development portfolio, but with the size, agility, and structure to respond rapidly when needed.
With a global team of experienced management, clinical research and scientific advisory specialists, we deliver precise clinical development solutions with expertise.
- First in Human/ Phase I
- Phase 1b / Phase 2a POC
- PK, food effect, drug-drug interaction
- Phase 2 & 3
- Post- Marketing
- IMP management
- Central laboratory support
Simbec-Orion is the CRO ready to take on the challenge. Whatever the indication or compound you are passionate about, wherever you are in your clinical development journey, we will act as an extension of your team to manage every element of your clinical trial.
Comprehensive support, integrated teams
Fabrice Chartier, CEO shares insights into what makes Simbec-Orion a unique Contract Research Organisation.
SOLUTIONS
Our clinical development solutions
We support clients with a range of functional and full-service clinical development solutions. Our wide range of clinical services include clinical trial management, clinical pharmacology, central laboratory support, and more – all customised to your project’s specific needs. Find out more about our clinical development services.
Central Laboratory Services

We are a CRO with in-depth experience in the most challenging therapeutic areas, including oncology, rare and orphan diseases, respiratory, dermatology, vaccines and anti-infectives.
With more than 25 years’ experience in the clinical research sector, we offer a comprehensive service you can trust – with an international reach. All with the highest level of quality, agility, and responsive customer service.
Clinical Pharmacology

As a full-service facility, we carry out all types of studies at our purpose-built site. 90% of our work is with Investigational Medicinal Products, in all drug delivery routes.
Clinical pharmacology is a key part of our CRO services, so if you need support managing a clinical trial, there are many ways our expertise can help.
Clinical Trial Management Services
Our expert biometrics team delivers accurate and adaptive clinical data management services that support the success of your drug development programme.
Conducting statistical analysis, planning, and reporting across all clinical phases, they will create a quality biostatistical framework that presents clinical trial data clearly and correctly for regulatory agencies.
THERAPY
Therapy areas in detail
The clinical research organisation with wide therapeutic experience, specialising in rare disease and oncology clinical development solutions.

What our
clients say about us
Simbec-Orion have great flexibility and a collaborative working style. Data management support for our study has been fantastic – the best data manager I have ever worked with. We have had a good Project Manager, and our statistics lead has been diligent, flexible and always delivered. Despite many changing parts, the team’s dedication has ensured all timelines were met.
CMO, UK pharmaceutical company, respiratory study